Testing
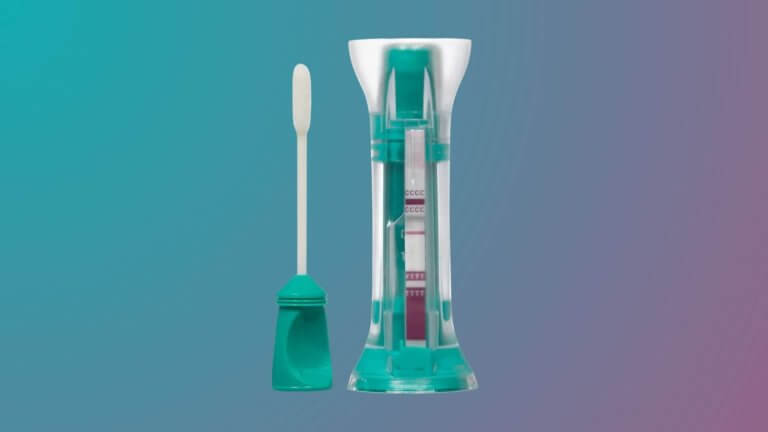
COVID-19 Rapid Antigen Self-Test, COVI-Go™, receives FDA Emergency Use Authorization for home use
Mologic Inc., the US subsidiary of Global Access Diagnostics (GADx), a leading developer of lateral flow and rapid diagnostic technologies, products and services, announced today that its proprietary COVI-Go™ SARS-CoV-2 Ag Self-Test received Emergency Use…