Innovation

Why Menstrual Blood Deserves a Second Look
Rethinking What We Throw Away There exists a biological resource so rich in proteins and stem cells it could unlock earlier diagnoses for conditions like endometriosis, PCOS, and even uterine cancer. Yet, this resource is…

5 Key Moments to Leverage Contract Research in Diagnostic Development
Delivering a lateral flow diagnostic test to market is a huge challenge. By leveraging contract research organisations (CRO) with expertise in development we can accelerate your success. In this post, we explore the key moments…

From Lab to Field: Progress in Crimean-Congo Haemorrhagic Fever Testing
In the ongoing fight against Crimean-Congo Haemorrhagic Fever (CCHF), Global Access Diagnostics (GADx) has joined forces with the Liverpool School of Tropical Medicine and international experts to develop a rapid diagnostic test (RDT) tailored for…
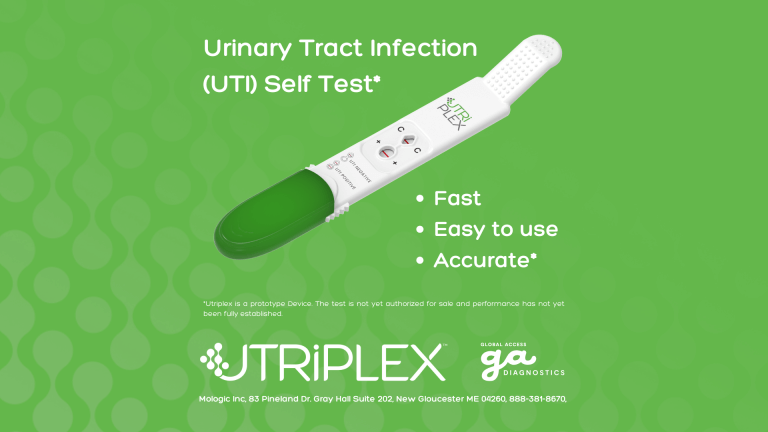
GADx Awarded Grand Prize for Maternal Health Innovation
Global Access Diagnostics (GADx) is thrilled to announce that we have been awarded the grand cash prize for our at-home urinary tract infection diagnostic by the National Institutes of Health’s (NIH) RADx Tech for Maternal…

Rapid Diagnostics for a Better World – POCT Leipzig Insights
Dr. Kevin Land, the Head of Innovative Research and Development at Global Access Diagnostics (GADx), delivered a compelling keynote at the prestigious POCT Leipzig conference on April 17, 2024. The event, hosted by the Fraunhofer…

GADx Contributes to TB Diagnostic Advancements
GADx is pleased to announce its significant contribution to a groundbreaking study published in PLoS One. The research, titled “Performance of Novel Antibodies for Lipoarabinomannan to Develop Diagnostic Tests for Mycobacterium tuberculosis,” showcases the collaborative…

GADx reintroduces IT LEISH, the rapid diagnostic test for visceral leishmaniasis
Global Access Diagnostics (GADx), a social enterprise prioritising equitable access to diagnostics, today announced the launch of IT LEISH, a rapid diagnostic test (RDT) for visceral leishmaniasis (VL). The company acquired the manufacturing rights for…
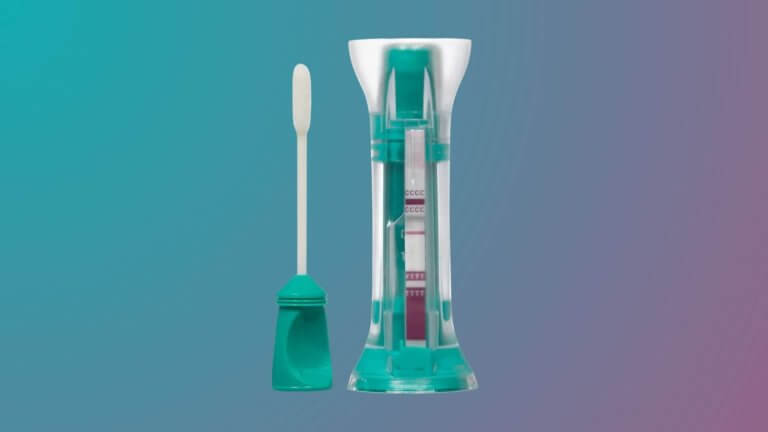
COVID-19 Rapid Antigen Self-Test, COVI-Go™, receives FDA Emergency Use Authorization for home use
Mologic Inc., the US subsidiary of Global Access Diagnostics (GADx), a leading developer of lateral flow and rapid diagnostic technologies, products and services, announced today that its proprietary COVI-Go™ SARS-CoV-2 Ag Self-Test received Emergency Use…

Detecting multiple diseases at once: EU-funded DIDIDA project develops a cost-effective mobile based solution in Africa
The Digital Innovations and Diagnostics for Infectious Diseases in Africa (DIDIDA) had its official kick-off at the Strathmore University. DIDIDA is an ambitious project to develop reliable, low-cost, mobile phone-connected tests to help detect multiple…
GADx formed to expand affordable access to advanced rapid diagnostic technologies
Global Access Health (GAH), a social enterprise dedicated to providing affordable medical products for global health, today formally introduced its first portfolio company, Global Access Diagnostics (GADx). GADx has been created to address gaps in…