
Our team of expert process development and technical transfer scientists bridge the gap between innovation and impact. We work with partners in low- and middle-income country settings to build local R&D and lateral flow assay manufacturing capacity. We can provide full support with our quality and regulatory expertise and clinical trials team.
Global Access Diagnostics is committed to ensuring our innovations and tools get to those who need them most. This commitment is underpinned by our status as a social enterprise, which allows us to provide access to life-saving tools. We always prioritise impact and access above profit.
CASE STUDIES & NEWS ARTICLES

Norbrook launches groundbreaking diagnostic test for Liver Fluke

GHLabs x GADx: PoC innovation in molecular diagnostics

GADx reintroduces IT LEISH, the rapid diagnostic test for visceral leishmaniasis
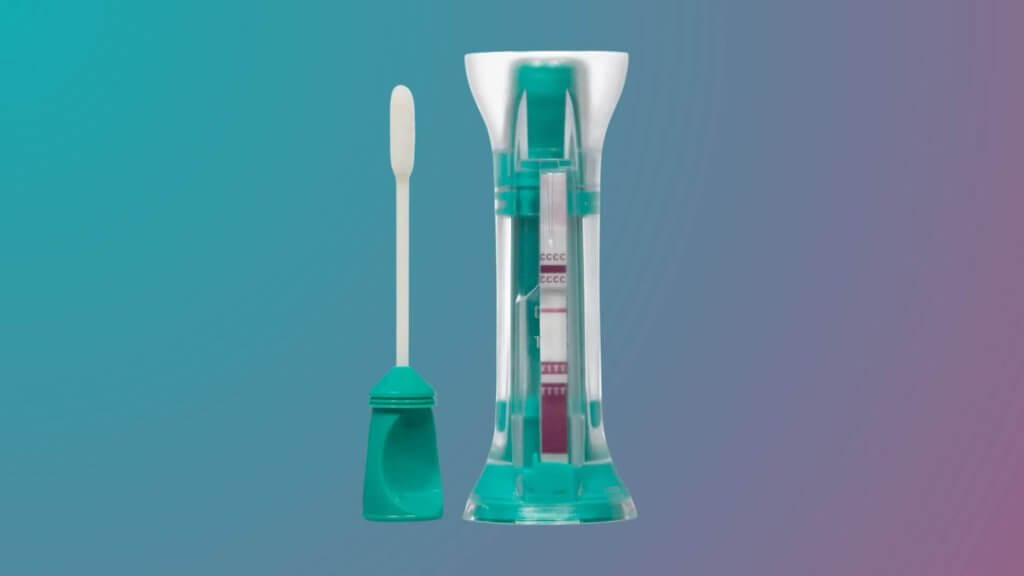
COVID-19 Rapid Antigen Self-Test, COVI-Go™, receives FDA Emergency Use Authorization for home use

GADx acquires manufacturing rights for IT LEISH rapid diagnostic test from Bio-Rad Laboratories
GET IN TOUCH
For sales inquiries please contact info@globalaccessdx.com
For technical support please contact technicalsupport@globalaccessdx.com
Frequent testing is invaluable and so it is vital to find ways to drive down the cost of kits and increase production levels. As a social enterprise, GADx can transform this process because it ‘breaks the link’ of ‘having to make profit for shareholders’.
Paul Davis
Co-founder of Mologic and pioneer of lateral flow diagnostics.