Ever wondered how we go about developing a lateral rapid test for the veterinary market ?
The developmental pathway to commercial product is broken into phases and entry will depend upon the stage of the assay. This can be as simple as starting with an idea, researching or perhaps you already have a test at Proof-of-Concept. At Global Access Diagnostics (GADx), a test can join or leave at any point along the pathway. For example, a test may stop if perhaps more funding or a change of scope is required. A phased flexible approach allows us to build work packages in bite sized chunks with regular Go / No Go’s to manage risk and change. After each phase has completed the progress of the assay is reviewed with appropriate documentation. Overall, a bespoke tailored approach to meet the developer, user and test needs.
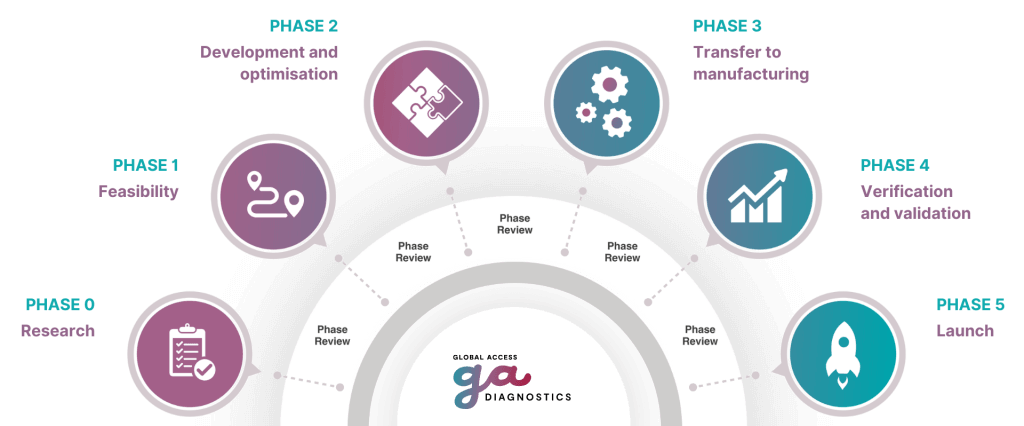
Case study of the individual stages
First steps in development will involve information gathering and consultation with the industry to understand:
- What is the test and the intended purpose.
- Is there really a need for the test.
- If yes, what the test needs to do to fulfil the industry requirement.
- Is there an industry gold standard or best practice the test can be evaluated against.
- A review of the market landscape to include:
- Who the end user is and their requirements.
- Market Adoption – will an industry leader / champion(s) be required.
- Is Certification required e.g. Global G.A.P., WOAH, part of a Supermarket Protocol
- Animal Host(s)
- Purpose of the test
- Sample type (s)
- Adoption requirements into the market
- Competitor tests – price, regions, claims, sensitivity and specificity, market share, unique selling points (if any), work flow and packaging.
- Market size
- Territories / Regions the test will be sold into and any regulatory requirement
- Route to Market.
- Acceptable cost of goods (COGS) for market uptake.
- A freedom to operate (FTO).
- Who will be responsible for the Product Design History, the Legal Manufacturer of the test and where will the test be manufactured.
- A review of the market landscape to include:
Whilst GADx as a test developer is able to support and evaluate the above will in test development require insight into:
- whether specific assay components are available e.g. antibodies, antigen and characterized samples.
- If yes, do they require a license for use or are they for research use only.
- If available, do they require license or a special laboratory facility / biological safety level e.g. Specified Animal Pathogens Order (SAPO) 2008, Plant Health Order 2015.
- Is the quality, concentration and supply of materials appropriate and available for development and if required, for scale up and commercialization activities.
- For Beta Phase testing / Field validation activities – who, how, where and by whom will this be carried out. Will ethical approval be required ?
In gathering this information, a Target Product Profile is formulated and from this a Design Inputs Requirement Document (DIRD) is created identifying the parameters of which the test device will be evaluated against both in terms of performance and market requirements. The DIRD is referenced throughout the product pathway to ensure the test device outputs meet the design inputs.
The below is given as a guide and can vary depending on the test and the commercial market aimed for:
Phase 1 – Feasibility
Entry to Phase 1 (Feasibility) considers the assay materials for the test device are available, and where license agreements are required for commercial application, that ‘in principle’ agreements / Heads of Terms are in place or identified as a risk if the project proceeds past Feasibility. Should development of suitable materials be required (Phase 0), GADx, as an innovation hub with expertise in biomarker discovery, immunogen development and targeted antibody development programs, is able to support development of these assay materials which may include
- epitope analysis of candidate structures
- provide expertise and support in smart immunogen design with conjugation processes and carrier molecules
- peptide array development and evaluation, synthesis and screening with Mass Spectrometry to evaluate structure and lyophilisation characteristics.
- design bespoke antibody pathways with evaluation of derived antibodies for binding kinetics suitable for LFA to include pairing dynamics using high throughput screening selections.
- evaluate small (ul) antibody volumes with gold conjugation by wet nitrocellulose paper strip assay to narrow down antibody candidates.

Figure 1. GADx enhance antibody development, discovery and selection process
Once the fundamental assay materials are in place, Phase 1 assay development can commence. First with evaluation in wet format. That is, the paper-based architecture is assembled and evaluated with antibody, homologous antigen and particle conjugate applied directly in liquid to evaluate binding potential at a test and control line. If appropriate binding events are achieved, initial work begins to determine if the design is feasible and to optimize the assay into a dry format to best meet test performance and cost considerations.
An Animal Health Diagnostic Test Development Plan (AHDTP) is written to provide an overview of the test and the proposed pathway, a risk management assessment of the test device and process is determined, and a review of the market and regulatory landscape made for the regions / territories the test device is to be sold into. The size of the market is critical in determining the product and manufacturing roadmap both in terms of batch size and the machinery required to produce the test devices. A low-mid volume manufactured batch size is in the region of 10,000 to 100,000 test devices and a high volume >100,000 test device batch size.
Potential designs, assay parameters, and sample panels are identified along with incoming QC of materials. As appropriate, a gold standard method or reference method is assigned. Experimental work is documented in lab notebooks and to complete the Phase:
- AHDTP is drafted and approved.
- Design Inputs Requirement Document (DIRD) is drafted and approved.
- A Design Trace Matrix (DTM) is created
- A Design History Folder (DHF) is created.
- End of Phase Technical Review with Phase 1 closure.
Phase 2 – Development and test device Optimization
During this phase the prototype test is further developed, refined and fully optimized with extended reference samples to ensure that the performance characteristics of the test are best suited to the intended purpose. For a veterinary diagnostic test with the aim of WOAH registration the following are listed as intended purpose examples:
- Contribute to the demonstration of freedom from infection in a defined population (country/zone/compartment/herd) (prevalence apparently zero):
- ‘Free’ with and/or without vaccination,
- Re-establishment of freedom after outbreaks
- Certify freedom from infection or presence of the agent in individual animals or products for trade/movement purposes.
- Contribute to the eradication of disease or elimination of infection from defined populations.
- Confirm diagnosis of suspect or clinical cases (includes confirmation of positive screening test).
- Estimate prevalence of infection or exposure to facilitate risk analysis (surveys, herd health status, disease control measures).
- Determine immune status of individual animals or populations (post-vaccination).
WOAH guidelines recommend for Phase 2 that a minimum of three well-defined reference samples are available, representing the target analyte ranging from high positive to negative (e.g. strong positive, weak positive and negative) to ensure the range detected by the assay is covered. The criteria for the selection of these reference samples should be determined ahead of Phase entry, with considerations as outlined below and availability known. For example:
- If using infected and uninfected animals from a population is the test being used in a particular geographical region or global ?
- Is the test targeting a particular breed, age, sex, nutritional status, pregnancy, immunological responsiveness, time from exposure to disease, reliability of a comparator’s method, prevalence in the herd or flock, disease incubation / latent period(s), stage of disease cycle, vaccination status, experimentally infected vs natural exposure (e.g. passively acquired antibody, active immunity elicited by vaccination or infection), co-infections, etc.
In addition, the reference samples will need to be evaluated in the same matrix as that used in the assay e.g. nasal fluid, hoof material, blood, plasma and or sera. In addition, any cross-reactants which may impact specificity, e.g. closely related pathogens, inclusion or exclusion of serotypes or pathotypes, antibodies to vaccinated animals) or interferences that may be present such as faecal, dirt, water, host material, therapeutics or anticoagulants should be evaluated for impact on the assay performance. These activities form part of the Phase 2 Pre Assay validation (Pre Verification) process and will represent key inputs listed in the DIRD. Evaluation of these inputs through testing of the device are to provide confidence at the end of Phase 2 that the test is fit to transition to Phase 3 with full validation activities in Phase 4.
Typical Phase 2 Assay Validation (Pre Verification) activities are:
- The setting of the positivity threshold(s) and the measuring / dyanamic range.
- Analytical specificity.
- Analytical sensitivity.
- Diagnostic performance (defined panel in sample matrices).
- Repeatability and reproducibility.
- Method comparison with gold or industry standard.
- Validation of the conditions of use*.
- Early accelerated stability under the prescribed storage and transport conditions.
* Assay Robustness activities (also known as Latitude testing or Ruggedness) evaluate the test against operational and environmental variables identified as critical in the DIRD, such as different operators and proficiency with repeatability activities at different times of the day and on different days, impact of temperature, humidity or other environmental factors and time to result. This process also enables the evaluation of different batches of material prepared at different times. At GADx we utilize this opportunity to derisk challenges that may arise when the test is scaled up at Tech Transfer (Phase 3) by enabling the R&D and Tech Transfer teams to work together in production of small pilot scale-up batches. These small pilot batch lots are generally used during Phase 2 Assay Validation (pre-verification) activities.
In addition to the above, it is advisable to plan and carry out pre Field Validation activities where the test is evaluated ‘in field’ with early end users of the technology and animal-side. For this, a draft instructions for use (IFU) is prepared.
A formal end of Phase 2 review is held where the outcomes of the Assay Validation activities are reported, reviewed against the DIRD, and the design traceability matrix (DTM) is updated to show traceability of the device outputs having met the design inputs. Samples and incoming Quality Control (QC) processes are identified for readiness of Phase 3. An initial estimate of the bill of materials (BOM) and cost of goods (COGS) are typically produced to ensure the commercial viability of the product in the marketplace. If the review is successful, the device can progress to Phase 3.
Phase 3 – Scale-up and manufacture
In Phase 3, the device and supporting materials are fully transferred to the Technology Transfer Team for scale-up and manufacture. A Technology Transfer Plan (TTP) is drafted and a scale-up Batch 0 is generated to support draft documentation, and if required, for full manufacture latitude testing. QC testing procedures and assay verification studies are drafted. Acceptance criteria, parameters and methods required for the device and process validation, that is to demonstrate the product can be consistently produced across batches, are reviewed. If successful scale up is demonstrated, the device validation batches (batches 1-3) are manufactured for process validation activities, and in readiness for Phase 4 Assay Validation (Verification) and Field Assay Validation activities. Draft packaging, labelling is developed and the IFU reviewed for final content. The batch manufacture process is documented in the AHDTP and TTP. The outputs from process validation input into the process failure mode and effects analysis (pFMEA).
The device packaging is in the final materials and configuration but may not contain the final branding. Packaging design and validation are typically conducted during Phase 3 and draft versions produced. The IFU must be at a stage suitable to allow for routine diagnostic use of the device and for the Field Validation studies. Supplier agreements, manufacturing SOPs, batch records and QC testing procedures are finalized, along with BOM and COGS.
Prior to performing stability studies, an Assay and Field Validation Plan (AFVP) is created which list the stability studies required and the acceptance criteria for each study type. Stability studies are initiated after the validation batches have been manufactured and passed QC. Depending on the complexity of the project, multiple reviews may be required during this phase. The completion of Phase 3 constitutes “Design Freeze”.
Phase 4 – Validation
Phase 4 is to confirm that the device conforms to the Design Input Requirements and is validated against the User needs Design Outputs. In evaluation, full Assay Validation (Verification) and Field Assay Validation activities are undertaken using the three validation batches manufactured in Phase 3. To support these activities, the IFU, device labelling and packaging are finalized. If possible with branding, along with any training materials. In addition, any Safety Data Sheets and a packaging validation report are prepared and made available.
Once the studies are complete, an Assay and Field Validation report is written summarizing the results, and if required, a risk-benefit analysis is carried out. The resulting documents are filed in the Design History File (DHF). For those tests which are classed as notifiable diseases and will submit an application to WOAH, an additional implementation stage will be required, as detailed in the WOAH Terrestrial Manual.
For Animal Health products placed on the market by GADx, a Post-Market Surveillance Plan (PMSP) can be prepared and will describe the process of collecting and logging any
complaints and reporting of these metrics.
The Design Trace Matrix is revised and a Phase 4 review meeting is held. Providing the deliverables are met by the supporting documentation, evidenced by the review panel and recorded in the DHF, the test can move to Phase 5.
Phase 5 – Product Launch and Post-Market Surveillance
As a diagnostic test deemed for use in the veterinary sector can in this ‘less‘ regulated space transition to Phase 5 either as a soft launch, with minor changes in design made prior to full launch, or direct to full commercial launch. For devices requiring registration approval, for example WOAH, once the device has received approval, any further changes will need to be agreed with the Regulatory / Registration Authority.
In a ‘less‘ regulated market, Post-Market Surveillance activities will be dependent on whether or how the Legal Manufacturer of the test wishes to implement and If registered, by the directives of the authorizing authority.
