We are constantly bombarded with headlines that ‘Bird Flu’ has the potential to become the next influenza pandemic. So why is this, should we be concerned and what preventative measures are being put in place. To answer these questions Global Access Diagnostics provides an introduction to the Influenza viruses, why avian influenza virus is of concern and what we are doing in terms of developing a rapid antigen test (RAT) which will support early diagnosis and surveillance.
Influenza viruses
Influenza viruses are divided into four types A, B, C and D and belong to the Orthomyxoviridae family. By microscopy Influenza A and B viruses are indistinguishable, spherical in shape and 80 to 120 nm in diameter. A and B have multiple surface antigens which undergo genetic and antigenic variation to maintain ‘viral fitness’, evade host immunity and cause reinfections. Both A and B circulate widely within the human population causing seasonal flu, with type A associated with global pandemics. Whilst the influenza viruses share a conserved and stable nucleoprotein, C and D are structurally distinct to A and B and present a variety of shapes. Influenza type C, with a single primary surface glycoprotein, is considered antigenically stable, is detected less frequently and usually causes mild infections. Influenza D primarily affects cattle and is not known to infect or cause illness in people
Avian Influenza A Virus (H5N1/Bird Flu). Source.
Influenza A and B
Influenza B (IBV), with no known animal reservoirs has diverged into two phylogenetic lineages which co-circulate within the human population causing approximately 25% of seasonal influenza cases (Source).
Influenza A circulates globally both within avian and mammalian hosts, and as a result of this broad gene pool, 130 influenza A virus (IAV) subtype combinations have been identified. Subtype classification is based upon the distribution and antigenicity of two surface glycoproteins: Hemagglutinin (HA) and Neuraminidase (NA). Each IAV will present in the region of 300 to 400 HAs and 40 to 50 NAs. To date, 18 subtypes of HA and 11 subtypes of NA have been identified, with potential for many more as a result of “reassortment”. This is when two influenza viruses infect a host at the same time and swap genetic information. Almost all subtypes of IAV, with the exception of H18N11 and H17N10 which originated in bats, are classified as avian influenza viruses (AIV).
AIV originates, and the genetic repertoire maintained, in wild water birds. In this form, the disease state exists as a low pathogenicity ‘pathotype’ with little or no clinical disease in those infected. Following introduction into poultry, IAVs may evolve and become highly virulent, causing high levels of mortality. Thus, AIVs can be sub-typed based on HA and NA classification and by pathotype. In addition, both IAV and IBV can be further broken down into different genetic clades and sub clades, although may not necessarily be antigenically different (Source).
Highly pathogenic AIV (HPAIV) can cross the species barrier from birds to mammalian species and cause sporadic human infections and/or fatalities. However, as of yet, have not been shown to have ability for sustained person to person transmission. In pigs the circulating AIV strains are AIV H1N1, H3N2 and H1N2 and in general outbreaks of the disease are within the pig population. Nevertheless, on occasion, a variant sub-type can cross from pigs to humans (Source). These ‘variant subtypes’ are different to those subtypes of AIV H1N1 and H3N2, which routinely circulate in people.
Pandemic Preparedness – Humans
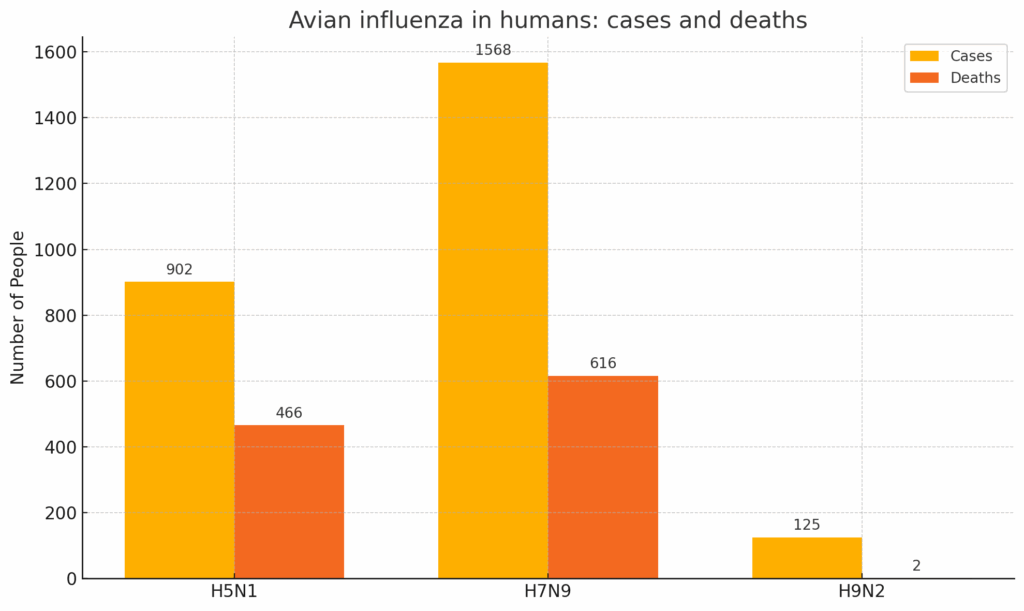
HPAI as a transboundary zoonotic disease impacts not only poultry, animal and human health but the wider communities around the world by threatening livelihoods, agricultural productivity, economies and ecosystems. Among the AIV HP sub-types of most concern are H5Nx (Source) and H9N2 AIVs. These are considered to have the greatest potential to cause a global influenza pandemic as they are widely prevalent in avian production, have a receptor binding shift towards a human like preference (Source) and are highly efficient in transmission. In unvaccinated chickens H5N1 infection latency is <0.25 days with a viral generation period of 1.3 days (Source). In addition, AIV HP H7N9 subtype is highly prevalent in poultry production and of pandemic concern (Source).
Of immediate concern are the evolving and geographically expanding AIV reassortment events leading to the emergence of HPAI sub types H5Nx 2.3 clade and subclades of the Goose/ Guangdong/1/96 (Gs/Gd) AIV lineage (Source). Thus far reported human cases have been associated with exposure to infected domestic birds and a few cases with exposure to infected wild birds, livestock and pets, indicating awareness for pandemic potential (Source).
Yet, no mutations in the receptor binding hemagglutinin (HA) protein have been identified that switch the binding preference of the HPAIVs from avian-like to human-like receptors. Transmission to humans remains a rare event with no reports of human-to-human transmission.
The majority of HP AIV H5Nx, H7 and H9 currently circulating are susceptible to all licensed antivirals for use in influenza infections. Only a few viruses have been observed that have mutations associated with resistance to antivirals, such as neuraminidase inhibitors (NAIs). (Source).
The World Health Organization maintains a list of all candidate vaccines for panzootic AIV (link) and currently there are no indications that the avian influenza viruses currently circulating differ antigenically from proposed candidate vaccine viruses for pandemic preparedness.
Current panzootic outbreak – Animals
Since 2020, changes in the Gs/GD HPAIV lineage have given rise to the emergence of the HPAIV H5N1 2.3.4.4b subclade which by migratory bird pathways has spread globally to areas of Asia, Europe and Africa, and extending into North America during 2021, Central and South America during 2022, and the Antarctic mainland in February 2024. This has led to H5N1 clade 2.3.4.4b viruses predominating over other AIV H5Nx clades and resulting in severe infection with disease and death in domestic, captive and wild birds and mammals, including deaths in seabird and sea mammal colonies (Source). Outbreaks of the disease have been reported on fur animal farms (mink, foxes, sable and raccoon dogs) and in dairy cows, goats and alpacas, and in some domestic pets (dogs and cats). In the UK, the world’s first case of Avian Flu (origin H5N1) is reported in sheep (Source). On-going cases reported in poultry, are at the highest levels recorded. However, there is no evidence that the viruses have adapted to spread between people. Similarly, studies evaluating risk of transmission through exposure to contaminated raw milk, where 2.3.4.4b of the H5N1 virus has been detected in cattle on more than 891 farms across sixteen states in the US, conclude that at present the circulating bovine HPAI viruses are unlikely to be able to efficiently transmit between humans (Source). Nevertheless, The UN advice is to drink pasteurized milk when possible, and if that is not available, of heating milk before consumption (Source).
Vaccination strategy to control panzootic outbreak
Whilst some countries like China and France vaccinate poultry, and the United States Department of Agriculture (USDA) has approved field safety studies for vaccine candidates designed to protect dairy cows from H5N1, many countries including the UK follow a no vaccination policy either for disease prevention or control (Source). The rationale being that current available vaccines have disadvantages in that, although able to reduce mortality, it’s possible that some vaccinated birds would be capable of onward transmission and negatively impact export trade. Similarly, this is the case in the US, where the export market is valued nearly $6 billion poultry export market (Source). Where vaccination is used with H5HA protein (subunit vaccine) or vector expressed H5HA, commercially available anti-nucleo protein (NP) ELISA test can be used to differentiate vaccinated from non-vaccinated animals. Whilst useful in settings where laboratory processing is available, will not if present, discriminate an H5N1 infection. Vaccination alone is not a strategy and requires an integrated approach with surveillance. Here, an effective, accessible and affordable rapid antigen test would prove helpful to a) identify a pan AIV infection, and if present, determine if H5N1 clade 2.3.4.4b or other H5, H7 and H9 subtypes. In support of these aims, Global Access Diagnostics with The Pirbright Research Institute are working together to realise this approach.
Improving Detection Sensitivity of Lateral Flow-Based Rapid Diagnosis of Emerging Avian Influenza H5Nx Clade 2.3.4.4b Viruses – Munir Iqbal presented a talk at the International Pandemic Sciences Conference, 1–2 July 2024, Oxford, UK. Our reflections.
Towards development of an on-farm lateral Flow test for Avian Influenza – Animals
AIV in poultry is shed in the faeces and respiratory secretions of infected birds. Transmission is either through direct contact or indirectly through contaminated feed and water, air, materials and equipment. AIV surveillance is carried out in different ways and to various extents across the world, by a range of bodies including the governmental and inter-governmental organizations, non-governmental organizations (NGOs), research institutions, regional organizations and large-scale commercial farms. In the UK, is a notifiable disease with testing carried out by the Animal and Plant Health Agency (APHA). Whether for export, import or quarantine purposes vets are required to submit with a form to request testing by APHA. Fees for testing are dependent on the sample number and test (Source). The diagnostic assay used will be determined on the purpose. For Avian influenza these can be divided into four areas: detecting the virus, its antigen, the genomic material or as a sero positivity test (antibodies produced in response to an AIV infection or vaccination). WOAH guidelines advise diagnosis is by isolation of the virus or by detection and characterisation of fragments of its genome. Whilst virus isolation confirms an infectious state of the individual or population of animals sampled from, Reverse Transcriptase Polymerase Chain Reaction (RT PCR) is often the preferred method being relatively quick, offering specificity, sensitivity, and with appropriate primers, enables subtyping of the virus. The ability to sequence the amplicon product generated provides genetic analysis and lineage to be determined. However, RT PCR is not able to determine if the virus is viable. Both approaches require laboratory processing and expertise with equipment and facilities to process the samples.
For both AIV virus antigen and sero positivity tests, enzyme linked immunoassay (ELISA) and lateral flow immunoassay (LFIA) can be deployed. Whilst ELISA requires a laboratory processing environment with dedicated equipment and a level of technical skill to operate, does allow large numbers of samples to be screened at one time. Some large poultry producers see value in routine screening for infectious diseases and antibody immune responses to vaccination and have invested in facilities to deploy ELISA with trained staff. For most however, this technology is unobtainable. LFIAs on the other hand, being accessible, affordable and easy to use, can readily be adopted on-farm by users with and without scientific expertise, to test poultry either at the individual level or at scale. Whilst for diagnosis of AIV infection, neither ELISA or LFIA can offer the specificity and sensitivity of RTPCR, can provide producers with an affordable and accessible tool to support monitoring of flock health on-farm.
The challenge in development of a suitable on-farm AIV test
LFIAs for AIV are commercially available for use with a range of poultry samples to include cloacal secretions, faecal and tracheal /oropharyngeal samples. However, limitations of use are many and range from in country legislation around use, an inability to discriminate HP from LP AIVs, or to determine the sub-type. In addition, a limited end user knowledge on the sample type to test for the poultry species concerned, the impact of environmental parameters and viral persistence in samples, and diagnostic test performance. Whilst there are LFIAs on the market which claim to identify a Pan AIV infection and to discriminate H5, H7 and H9 sub-types are considered to lack the desired sensitivity required for early intervention as part of a disease management strategy. Global Access Diagnostics with The Pirbright Research Institute are working together to mitigate this and to develop a suite of first generation rapid LFIA tests for the poultry industry to detect AIV in the sample matrices described at an LOD in the region of <1000 AIV plaque forming units / ml. If present, selectively identify if a H5, H7 or H9 AIV. The H5 test is specifically designed to include recognition of the circulating HP H5Nx sub types to include H5N12.3.4.4b. A second generation of tests to incorporate a DIVA H5 AIV and other poultry diseases to include Newcastle disease virus (NDV) and Infectious bronchitis (IB).
Further reading: An Introduction to Lateral Flow and Product Development (Veterinary and Agriculture)

