A Lateral Flow Immunoassay (LFIA), also known as an immunochromatographic test strip, is a rapid, simple paper based diagnostic test platform used to detect specific analyte(s) / antigen in liquid samples. First patented in the early 1980s, this technology entered the market in the late 1980s as an affordable, over-the-counter home pregnancy test. Professor Paul Davis, former Chief Scientific Officer and founder of Mologic Ltd (trading as Global Access Diagnostics, GADx), an end-to-end lateral flow development company, is one of the original scientists (Davis, May and Prior – 1989), whose work led to the creation of UniPath and the Clearblue brand of home pregnancy tests.
Over the past 40 years, the acceptance and value of LFIAs has gained momentum globally, as evidenced during the Covid pandemic. Applications now extend far beyond clinical diagnosis into veterinary medicine, agriculture, environmental testing, bioterrorism detection, industrial use and in food safety. In 2022, the global lateral flow market was valued at USD 10.69 billion and is expected to grow at a compound annual growth rate (CAGR) of 4.26% from 2023 to 2030, with greatest market share held by the US at 25% (Source).
In its original form the LFIA relies on harnessing specific antibody binding events to support generation of a test and control line. Different assay choreographies can be demonstrated but are generally based around an antibody sandwich assay (Figure 1) or an inhibition / competitive assay format (Figure 2).

Figure 1. Workflow of a simple antibody sandwich lateral flow with a positive outcome

Figure 2. Workflow of a simple inhibition / competitive lateral flow assay with excess target analyte / antigen present in the sample (strong positive result), with low target analyte / antigen present in the sample (weak positive) and target analyte / antigen absent (negative result)
The advent of molecular diagnostic assays has enabled the test strip to evolve integrating both immunological and nucleic binding events (nucleic lateral flow immunoassay (NALFIA, Figure 3).

Figure 3. Generic workflow of a simple NALFIA assay with a positive outcome and an internal amplification control
Later, the development of nucleic lateral flow formats (NALF), which in the absence of antibodies or immunoreagents, relies solely on binding / hybridization of complementary nucleic oligos tagged with visual particles such as gold or fluorescein. Often with the capture oligo anchored to the membrane with an integrated linker such as biotin to bind a complementary marker such as streptavidin (Figure 4).

Figure 4. Generic workflow of a simple NALF assay with a positive outcome
The LFA has demonstrated both sensitivity and specificity at an affordable price, usability in low-resource settings without electricity, laboratory equipment or trained operators. The test is simple to run, particularly LFIA tests with minimal or no upfront sample preparation. The practicality of which was shown with Covid-19 testing, where a nasal swab was taken and inserted directly into pre-prepared liquid vial, mixed by inverting the sample tube several times, and applied directly to the LFIA. Similar simplicity applies to other sample matrices such as blood (Source). In these tests, blood droplets are collected and directly applied to the LFA sample pad, flowing through overlapping material zones by capillary action to an absorbent pad at the end of the test strip. As the sample flows across the device molecules interact to form a test and control line. The control line indicates the test has run correctly. Depending on the test parameters, results are visible between 3 and 30 minutes.
The primary visual label used in LFA is gold nanoparticles (AuNPs) with a typical size range 60 to 80nm in diameter, bound to the immuno or molecular complexes to form a pink / red line or spot. Dyed latex particles are also common, while paramagnetic particles and carbon are used less frequently due to scale up and manufacturing limitations. The formation of a test line is dependent on whether a target analyte is present. The test line intensity can be read either by eye or using a reader (Figure 5)

Figure 5. Visual intensity read of the test and control line with a score card (A) with a Phone like App (single source code with test line processing via Cloud link) with a phone App (C) and (D) using a portable digital reader (opTricon Cube reader shown).
In the race to improve detection limits, LFA technologies and reagents are continuously advancing with measurement methods, such as semi-conductor particles, photoacoustic techniques, Surface-enhanced Raman Spectroscopy (SERS) integrated with use of smartphones, digital thermal readers, and fluorescence resonance energy transfer (FRET) readers Lateral Flow Assays Market Analysis Report 2025-2030:
The LFIA can be qualitative or semi-quantitative and in its simplest form provide a single test and control line (simplex LFA). As a multiplex test, it can analyse multiple analytes simultaneously in a sample, whilst under the same test conditions. The format of a multiplex test can vary from multiple dots or test lines on a single LFA (Figure 6A) test strip or across multiple test strips in a single device (Figure 6B). Similarly, the strip can be run vertically or horizontally with or without a housing.

Figure 6. Multiplex test on a single lateral flow strip (A) and as a two-strip test from a single swab sampling device within a one-step sealed vertical housing (B).
The Stages of LFA Development in a ‘less regulated’ Market (Veterinary & Agriculture)
While clearly defined regulatory and development product pathways exist for human in vitro diagnostic tests, this is currently not the case for the veterinary and agricultural diagnostic test industry. Plant pathogen diagnostic tests, despite being a multi-billion pound industry, has no formal regulatory framework or validation process. “Fitness for intended purpose” is advised but formalized guidelines have not yet been established. (Source).
For animal veterinary diagnostics, regulatory efforts and oversight of veterinary medical devices can be more formalized but are not globally harmonized. In the EU, at the time of writing, there is no regulation for diagnostic devices developed solely for veterinary animal use. This allows European Union Member States and Economic European Area (EAA) countries (Norway, Iceland and Liechtenstein) to apply their own rules. For product registration, certification and/or placing veterinary diagnostic test devices on the market, companies should address the relevant national authorities of the member or EAA states concerned.
In general, there is no requirement for a veterinary medical diagnostic to be CE-marked or meet the requirements for the CE marking. It is the responsibility of the production site releasing the batch of finished product to guarantee the quality system compliance, proper labelling and product safety during EU distribution. Trials in veterinary clinics by qualified veterinary surgeons can generate safety data and bibliographic references may be used for promotional literature. However, this regulatory landscape may change, as both the EU and UK are evaluating new legislation around Diagnostic Analyses for Animal Testing.
In the US, at the time or writing, there is no formal requirement for FDA approval of medical devices used exclusively for animals (veterinary devices). The FDA does not require submission of a 510(k), PMA, or any pre-market approval for veterinary devices intended for animal use. (Source)
For the rest of the world, legislation does not correlate to market size or economic strength of the country. The World Organization for Animal Health (WOAH) has to some extent tried to overcome these inconsistencies. An inter-governmental organisation with 182 member countries set up for improving animal health worldwide, provides a Certification and Proficiency scheme with International Reference Laboratories and Standards to support animal diagnostic tests (Source) also provides a procedure for the registration of diagnostic kits (link) to provide countries with a list of tests certified by the WOAH as fit for purpose (link).
In diagnostic test development for this sector, GADx accordingly, has developed a defined phased product pathway and for Veterinary LFIA products is based on the standards, guidelines and recommendations issued by WOAH (link) with specific reference to Chapters 2.2.1.,2.2.2. and 2.2.3.
For other Agricultural, Food and Environmental tests, the GADx Phased approach is adapted to fit the purpose, and market requirements. The developmental pathway to commercial product is broken into phases (Figure 7) and entry will depend upon the stage of the assay for the desired outcome.
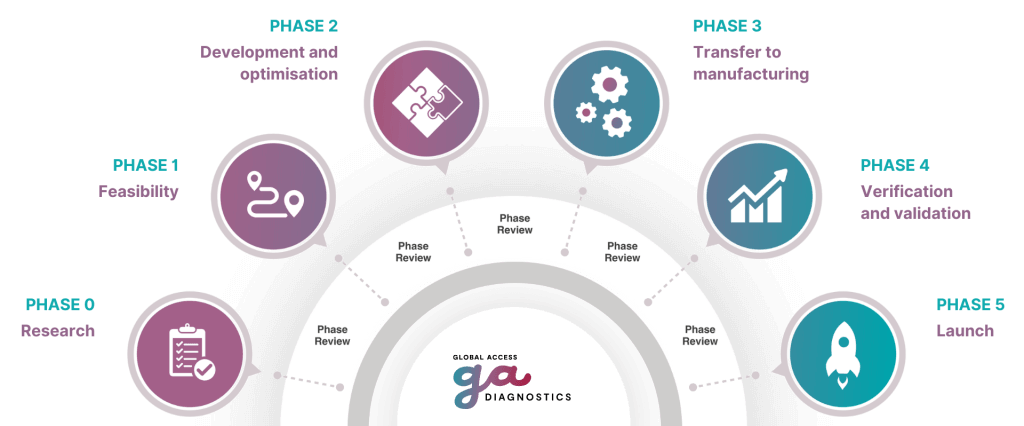
Figure 7. GADx lateral flow product development pathway
Further reading: Bird Flu in Focus: The Science, the Risk, and the Road to Preparedness
