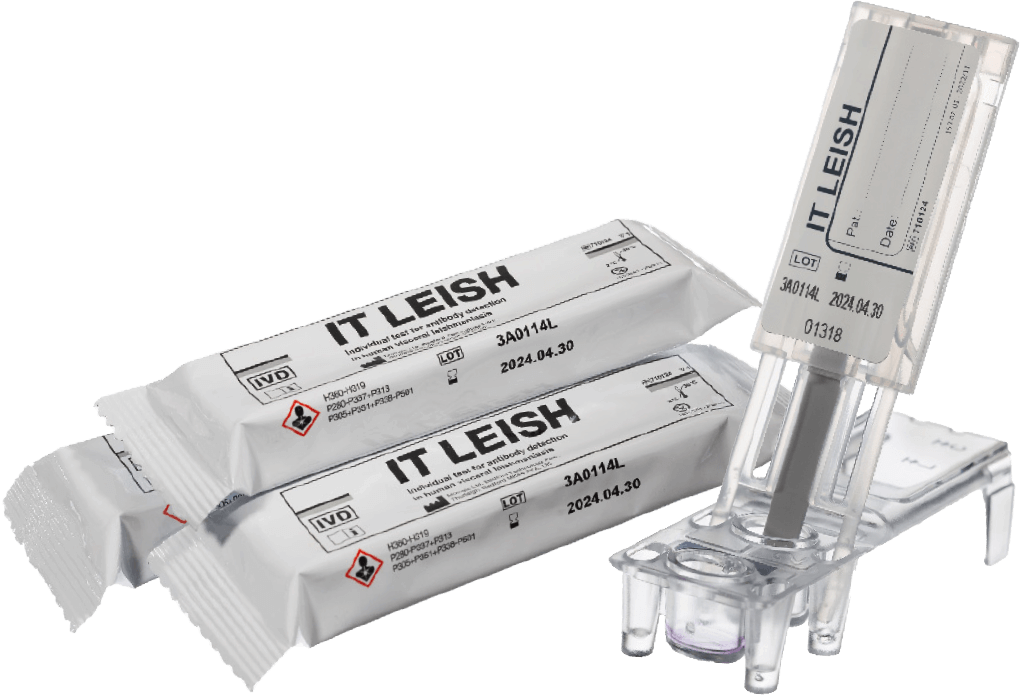
IT LEISH: Individual test for antibody detection in human visceral leishmaniasis
- Rapid test for detection of Leishmania spp. specific antibodies in serum or capillary whole blood during infection
- Manually performed, visually read, single-use, for professional use only
- Results in 10 minutes (with serum)
Downloads
GET IN TOUCH
For sales inquiries please contact info@globalaccessdx.com
For technical support please contact technicalsupport@globalaccessdx.com

Botrytis Alert is a field-side, quick and easy to use low-cost test to measure Botrytis in the air and plant.
- Botrytis Alert is the first step to reduce crop and post-storage harvest losses
- Botrytis Alert can be used to help inform decision making and drive early intervention to prevent polycyclic disease epidemics and post-storage rots.
- Botrytis Alert: Monitor, Act, Treat, Control and Harvest (MATCH)

- This product has not been FDA cleared or approved, but has been authorized by FDA under an EUA;
- This product has been authorized only for the detection of proteins from SARS- CoV-2, not for any other viruses or pathogens; and,
- The emergency use of this product is only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostics for detection and/or diagnosis of COVID-19 under Section 564(b)(1) of the Federal Food, Drug and Cosmetic Act, 21 U.S.C. § 360bbb-3(b)(1), unless the declaration is terminated or authorization is revoked sooner.
Frequent testing is invaluable and so it is vital to find ways to drive down the cost of kits and increase production levels. As a social enterprise, GADx can transform this process because it ‘breaks the link’ of ‘having to make profit for shareholders’.
Paul Davis
Co-founder of Mologic and pioneer of lateral flow diagnostics.